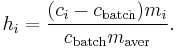Homogeneity and heterogeneity
Homogeneity and heterogeneity are concepts relating to the uniformity or lack thereof in a substance. A material that is homogeneous is uniform in composition or character; one that is heterogeneous lacks uniformity in one of these qualities.[1][2][3]
The concepts are applicable to every level of complexity, from atoms to populations of animals or people, to galaxies. Hence, a substance may be homogeneous on a larger scale, compared to being heterogeneous on a smaller scale within the same substance. This is known as an effective medium approach, or effective medium approximations.[4][5]
Contents |
Heterogeneity
Heterogeneity is the state of being heterogeneous. It is the nature of opposition, or contrariety of qualities. Pertaining to the sciences, it is a substance that is diverse in kind or nature; composed of diverse parts. In other words, it is composed of dissimilar parts, hence the constituents are of a different kind. The parts (or constituents) are connected, and of a conglomerate mass, and viewed in respect to the parts of which it is made up.[1][2]
Various disciplines understand heterogeneity, or being heterogeneous, in different ways. For example:
- In physics, it is understood as having more than one phase (solid, liquid, gas) present in a system or process.
- In chemistry, a heterogeneous material consists of either or both of a) multiple states of matter or b) hydrophilic and hydrophobic substances in one mixture; an example of the latter would be a mixture of water, octane, and silicone grease.
- With information technology (see:Heterogeneous computing) it means a network comprising different types of computers, potentially with vastly differing memory sizes, processing power and even basic underlying architecture. Alternatively, a data resource with multiple types of formats.
- In sociology it may refer to a society or group that includes individuals of differing ethnicities, cultural backgrounds, sexes, or ages.
- Rocks (geology) are inherently heterogeneous, usually occurring at the micro-scale and mini-scale.[4]
Homogeneity
Homogeneity is the state of being homogeneous. Pertaining to the sciences, it is a substance where all the constituents are of the same nature; consisting of similar parts, or of elements of the like nature. For example, homogeneous particles, homogeneous elements, homogeneous principles, or homogeneous bodies; or (algebra) possessing the same number of factors of a given kind as with a homogeneous polynomial.[3]
Mathematics
In mathematics, homogeneous may refer to:
- Homogeneous differential equation
- Homogeneous distribution
- Homogeneous function
- Homogeneous polynomial
- Homogeneous space
Chemistry
A heterogeneous mixture is a mixture of two or more compounds. Examples are: mixtures of sand and water or sand and iron filings, a conglomerate rock, water and oil, a salad, trail mix, and concrete (not cement).[6] During the sampling of heterogeneous mixtures of particles, the variance of the sampling error is generally non-zero. Gy's sampling theory [7] quantitatively defines the heterogeneity of a particle as:
where 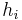 ,
, 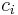 ,
, 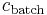 ,
, 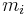 , and
, and 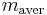 are respectively: the heterogeneity of the
are respectively: the heterogeneity of the 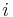 th particle of the population, the mass concentration of the property of interest in the
th particle of the population, the mass concentration of the property of interest in the 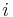 th particle of the population, the mass concentration of the property of interest in the population, the mass of the
th particle of the population, the mass concentration of the property of interest in the population, the mass of the 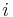 th particle in the population, and the average mass of a particle in the population.
th particle in the population, and the average mass of a particle in the population.
Homogenization is the process of causing a heterogeneous mixture to become homogeneous, as is done with the making of homogenized milk.
Homogeneous and heterogeneous reactions
Homogeneous reactions are chemical reactions in which the reactants are in the same phase, while heterogeneous reactions have reactants in two or more phases. Reactions that take place on the surface of a catalyst of a different phase are also heterogeneous. A reaction between two gases, two liquids or two solids is homogeneous. A reaction between a gas and a liquid, a gas and a solid or a liquid and a solid is heterogeneous.
A mixture can be determined to be homogeneous when everything is settled and equal, and the liquid, gas, object is one color or the same form. Various models have been proposed to model the concentrations in different phases. The phenomena to be considered are mass rates and reaction rates. Surface area affects the reaction rate of heterogeneous reactions but not homogeneous reactions.
Biology
Genetic heterogeneity refers to multiple origins causing the same disorder in different individuals. Heterogeneity of ion channels means diversity of different types of channels serving a specific kind of current, e.g. by channels with different constitutive subunits.[8]
Economics
Heterogeneity of the units under study (consumers, firms, products, etcetera) causes complications in economic analysis. Unobserved heterogeneity complicates econometrics because it may cause statistical bias in estimated coefficients. It complicates the theoretical analysis of economic models, since the model must be enlarged with more equations or variables to take into account the effects of the differences across units.
See also
References
- ^ a b "Webster's Revised Unabridged Dictionary (1913 + 1828)" (Part of this paragraph is public domain material copyright 1828 and 1913). Heterogeneity. The ARTFL Project, University of Chicago. September 2010. http://machaut.uchicago.edu/?resource=Webster%27s&word=Heterogeneity&use1913=on&use1828=on. Retrieved 2010-09-10.
- ^ a b "Webster's Revised Unabridged Dictionary (1913 + 1828)" (Part of this paragraph is public domain material copyright 1828 and 1913). Heterogeneous. The ARTFL Project, University of Chicago. September 2010. http://machaut.uchicago.edu/?action=search&word=heterogeneous&resource=Webster%27s&quicksearch=on. Retrieved 2010-09-10.
- ^ a b "Webster's Revised Unabridged Dictionary (1913 + 1828)" (This is public domain material copyright 1828 and 1913). Homogeneous. The ARTFL Project, University of Chicago. September 2010. http://machaut.uchicago.edu/?resource=Webster%27s&word=homogeneous&use1913=on&use1828=on. Retrieved 2010-09-10.
- ^ a b Guéguen,, Yves; and Palciauskas, Victor (May 1994). Introduction to the physics of rocks. Princeton University Press. pp. 53–72 (Chapter 3). ISBN 9780691034522. http://books.google.com/?id=fCP5qyRyX-oC&pg=PA53&dq=heterogeneous+physics#v=onepage&q=heterogeneous%20physics&f=false.Google Books preview download available
- ^ Shadrivov, Ilya V.; Kozyrev, AB; Van Der Weide, DW; Kivshar, YS (2008-11-24). "Nonlinear magnetic metamaterials" (Introduction section. Free PDF download). Optics Express 16 (25): 20266–71. Bibcode 2008OExpr..1620266S. doi:10.1364/OE.16.020266. PMID 19065165. http://assets0.pubget.com/pdf/19065165.pdf. Retrieved 2009-11-26.
- ^ Gamow, George (April 1967). "Chapter VI, "Descending Staircase"" (Mass market paperback). One Two Three... Infinity (Bantam Science and Mathematics, 5th printing ed.). Bantam. p. 117. "[Clam chowder] represents a nice example of what is known as a heterogeneous material."
- ^ Gy, P (1979) Sampling of Particulate Materials: Theory and Practice, Elsevier: Amsterdam, 431 pp.
- ^ Vicini S (April 1999). "New perspectives in the functional role of GABA(A) channel heterogeneity". Mol. Neurobiol. 19 (2): 97–110. doi:10.1007/BF02743656. PMID 10371465.
External link
- The following cited pages in this book cover the meaning of "homogeneity" across disciplines Morris, Christopher G. (1992). Academic Press Dictionary of Science and Technology. Academic Press Inc.. pp. 1039, 1040. ISBN 0122004000. http://books.google.com/?id=nauWlPTBcjIC&pg=PA1039&dq=Homogeneity+in+physics#v=onepage&q=Homogeneity%20in%20physics&f=false..